Robust and resource-optimal dynamic pattern formation of Min proteins in vivo
 Understanding the physical underpinnings of dynamic intracellular organization remains central to elucidating spatial regulation in cells. Here, we present a comprehensive experimental and theoretical study of the Min protein system in Escherichia coli, revealing how robust and resource-optimal dynamic pattern formation underpins accurate division site selection. Using engineered strains with tunable expression of MinD and MinE, we construct an in vivo phase diagram of Min oscillations and uncover that pattern formation persists over a broad range of protein concentrations and physiological growth states.
Understanding the physical underpinnings of dynamic intracellular organization remains central to elucidating spatial regulation in cells. Here, we present a comprehensive experimental and theoretical study of the Min protein system in Escherichia coli, revealing how robust and resource-optimal dynamic pattern formation underpins accurate division site selection. Using engineered strains with tunable expression of MinD and MinE, we construct an in vivo phase diagram of Min oscillations and uncover that pattern formation persists over a broad range of protein concentrations and physiological growth states.
Our experiments show that wild-type expression levels lie close to the minimal concentrations required for pattern formation, suggesting evolutionary optimization for resource-efficient functionality. Moreover, the phase diagram reveals two distinct no-pattern regimes at extreme [MinD]/[MinE] ratios—corresponding to filamentous and minicell phenotypes—highlighting the importance of balanced stoichiometry for robust Min dynamics.
A central insight arises from the integration of a reaction–diffusion model incorporating a conformational switch in MinE, enabling it to toggle between a reactive and a latent state. This switch mechanism buffers protein levels and extends the range of parameters supporting dynamic oscillations, reconciling the observed robustness with theoretical expectations. Numerical simulations of this MinE-switch model accurately reproduce experimental phase diagrams, including the transition between travelling and standing wave patterns in filamentous cells.
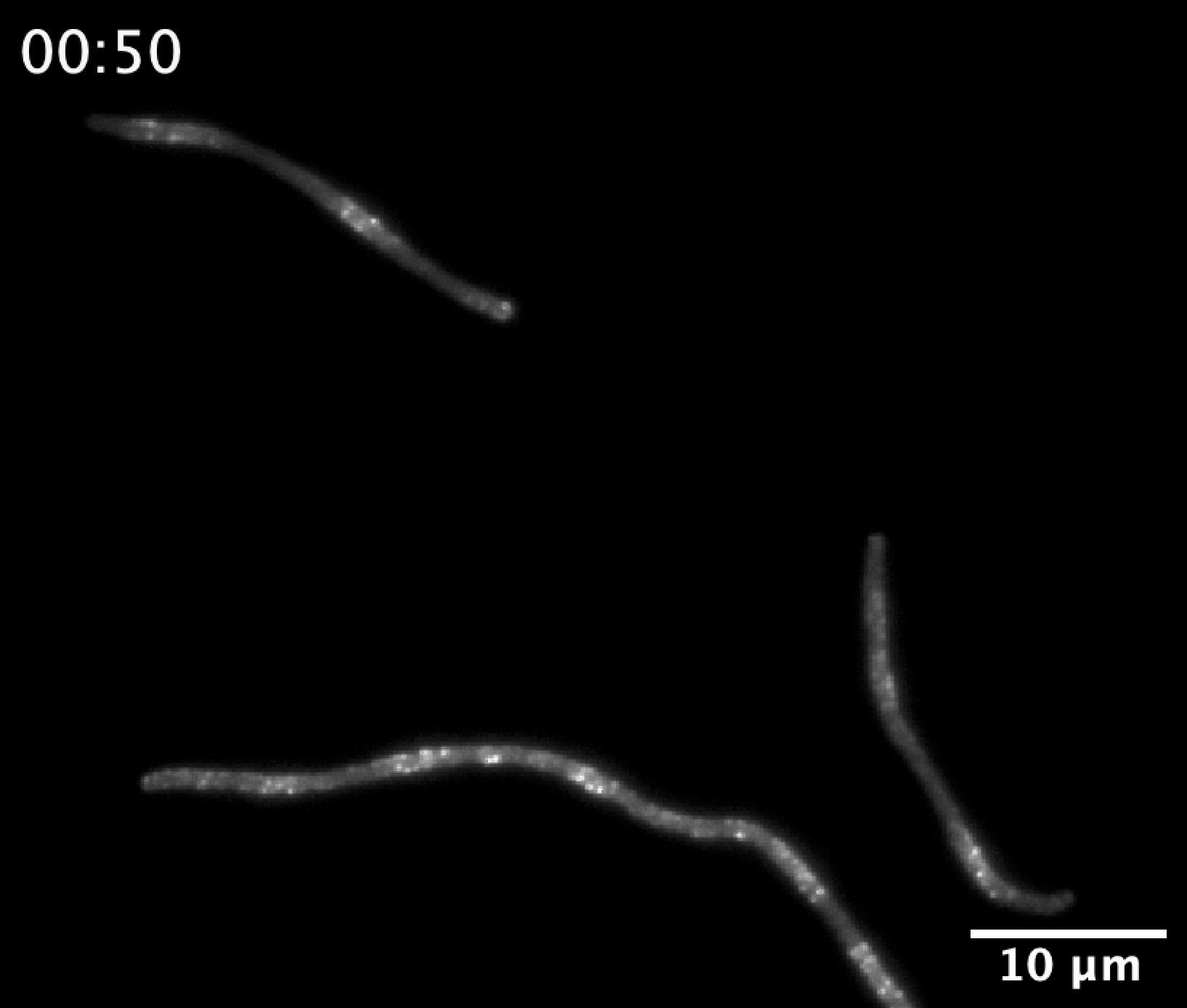
Strikingly, the Min patterns maintain an invariant wavelength of ~8 µm across more than tenfold changes in protein concentration and growth rate, a feature captured by the model and indicative of intrinsic length-scale selection. This invariance coincides with the upper length scale of E. coli cells, suggesting that spatial patterning by the Min system is not only robust but tightly coupled to cellular morphology and division timing.
Taken together, this work provides a unifying framework that links gene expression, cell physiology, and nonlinear biochemical dynamics. It highlights conformational switching as a general design principle for robustness in self-organized cellular systems and illustrates how quantitative modeling and synthetic perturbations can reveal the operating principles of spatiotemporal pattern formation in living cells.

